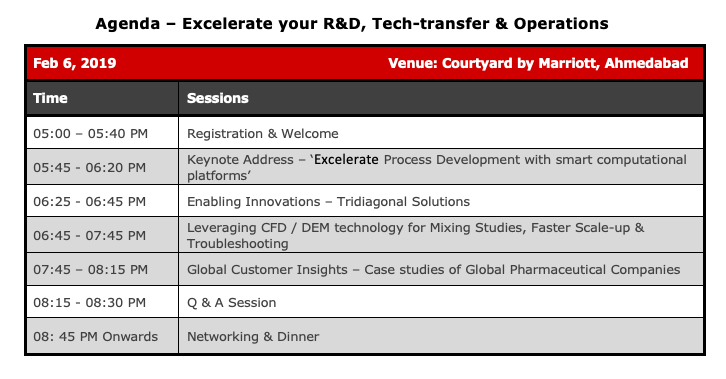
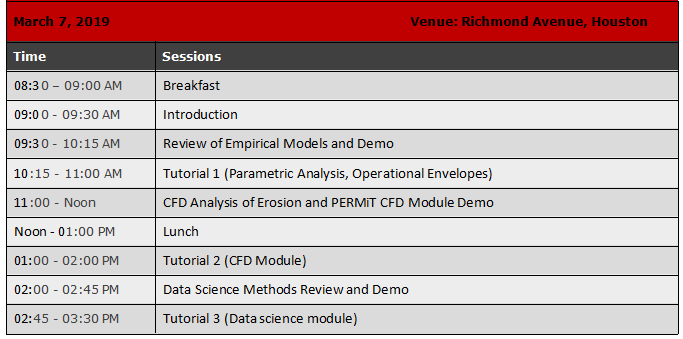
Achieve Reliable Process Technology Transfer With Fundamental Process Understanding
- Save to Calendar
- 28/01/2021 - 11:00 am - 11:45 am CST
Pharma processes are among the more complex processes in the chemical industry. Over the past decade or so, the traditional “quality-by-testing” approach of trial-and-error is being consciously replaced, partly or fully, by a more scientific approach across all aspects of pharmaceutical engineering – discovery, development and manufacturing. This “Quality by Design (QbD)” paradigm – which is a risk and fundamentals based approach to process development and transfer – makes it imperative that the entire development lifecycle be carried out in a systematic, science-based and quality risk-driven manner, so that the quality of the final product is no longer left to chance.
The central tenet of QbD is “process understanding” – a term used by the US FDA to describe the degree of knowledge that an organization has acquired on its product and the pertinent manufacturing processes. Process understanding is not a “nice-to-have”. It is actually critical to achieve a state of operations where things are done right the first time, every time. In fact, with good process understanding, an organization stands to reap a number of impactful business benefits such as reduced time to market, better use of resources, elimination of wastage and perhaps most importantly, a no-surprises path to regulatory approval.
In this webinar (the first of a series), we will show how science-driven analysis and data-driven modeling can be combined to develop a systematic and economically feasible workflow to get process technology transfer right first time, every time.
| << | Apr 2024 | >> | ||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 | 1 | 2 | 3 | 4 | 5 |
-
Register Now
Achieve Reliable Process Technology Transfer With Fundamental Process Understanding
28/1/202111:00am CST45 minutesPharma processes are among the more complex processes in the chemical industry. Over the past decade or so, the traditional “quality-by-testing” approach of trial-and-error is being consciously replaced, partly or fully, by a more scientific approach across.
In this webinar (the first of a series), we will show how science-driven analysis and data-driven modeling can be combined to develop a systematic and economically feasible workflow to get process technology transfer right first time, every time.