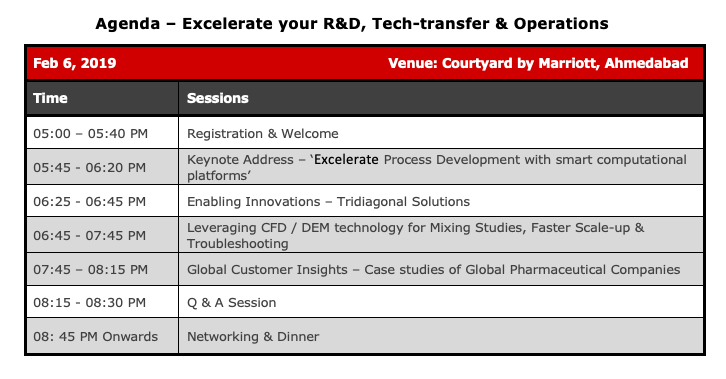
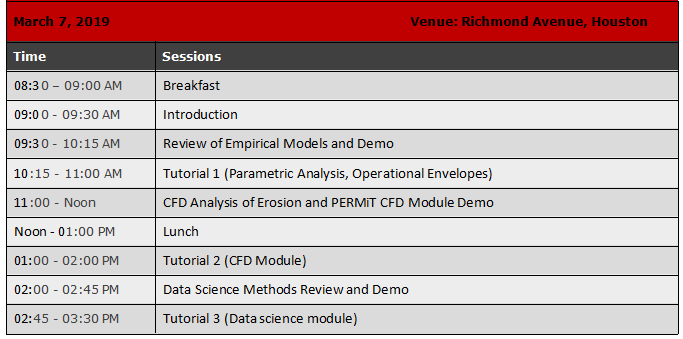
Bioprocess Understanding For Reliable Development & Transfer : The Role Of Mixing
- Save to Calendar
- 25/03/2021 - 11:00 am - 12:00 pm CDT
- Fundamental process understanding plays a critical role in biopharmaceutical drug development and process transfer. Increasingly sophisticated upstream/downstream processes and the introduction of novel technologies such as single-use/disposable equipment has served to further elevate this criticality. One process consideration that is common to many key USP and DSP steps is to ensure good mixing in a vessel. This is true irrespective of the nature of the biopharmaceutical product or the process step in question – buffer/media prep, cell culture, formulation, viral clearance/inactivation etc.In this webinar, we consider this critical role that mixing plays across the spectrum of bioprocess steps in some detail. We show how a thorough understanding and characterization of mixing in bioreactors and other such vessels can ensure reliable process development, scale up and transfer. We will pay particular attention to mixing in microbial cell cultures vs animal/plant cell cultures. The importance of mixing in other process steps such as media prep, buffer prep, formulation etc. will also be treated in some detail.Mixing characterization using experimental and computational methods will also be presented. We will start by describing the appropriate set of process metrics to characterize bioprocess mixing before proceeding to a discussion on the specific methods.In keeping with the current trends in the industry, illustrative examples of engineering characterization will be provided using COTS single-use systems from major vendors (Sartorius, Millipore, Pall to name a few).
| << | Apr 2021 | >> | ||||
| M | T | W | T | F | S | S |
| 29 | 30 | 31 | 1 | 2 | 3 | 4 |
| 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 26 | 27 | 28 | 29 | 30 | 1 | 2 |
-
Register Now
Bioprocess Understanding For Reliable Development & Transfer : The Role Of Mixing
25/3/202111:00am CDT1 hourFundamental process understanding plays a critical role in biopharmaceutical drug development and process transfer. Increasingly sophisticated upstream/downstream processes and the introduction of novel technologies such as single-use/disposable equipment has served to further elevate this criticality. One process consideration that is common to many key USP and DSP steps is to ensure good mixing in a vessel. This is true irrespective of the nature of the biopharmaceutical product or the process step in question - buffer/media prep, cell culture, formulation, viral clearance/inactivation etc.
In this webinar, we consider this critical role that mixing plays across the spectrum of bioprocess steps. We show how a thorough understanding and characterization of mixing in bioreactors and other such vessels can ensure reliable process development, scale up and transfer. We will pay particular attention to mixing in microbial cell cultures vs animal/plant cell cultures. The importance of mixing in other process steps such as media prep, buffer prep, formulation etc. will also be treated in some detail.
Mixing characterization using experimental and computational methods will also be presented. We will start by describing the appropriate set of process metrics to characterize bioprocess mixing before proceeding to a discussion on the specific methods.
In keeping with the current trends in the industry, illustrative examples of engineering characterization will be provided using COTS single-use systems from major vendors (Sartorius, Millipore, Pall to name a few).